Glycolic acid (or hydroxyacetic acid) is the smallest α-hydroxy acid (AHA). This colorless, odorless, and hygroscopic crystalline solid is highly soluble in water. It is used in various skin-care products. Glycolic acid is found in some sugar-crops.
1. Properties
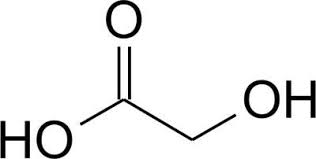

Name:Glycolic acid
EINECS:201-180-5
Molecular Formula:C2H4O3
CAS Registry Number:79-14-1
Synonyms:Hydroxyacetic acid; Glycolic acid solution; glycollic acid; Hydroxyethanoic acid
InChI:InChI=1/C2H4O3/c3-1-2(4)5/h3H,1H2,(H,4,5)
Appearance:Light yellow to amber liquid
Molecular Weight:76.05
Density:1.27
Boiling Point:113 °C
Melting Point:10 °C
Flash Point:128.7 °C
Refractive index:n20/D 1.424
Solubility:H2O: 0.1 g/mL, clear
Stability:Stable. Incompatible with bases, oxidizing agents and reducing agents.
2. Uses
Glycolic acid can be used in various skin-care products.Due to its excellent capability to penetrate skin.
Glycolic acid is also a useful intermediate for organic synthesis, in a range of reactions including: oxidation-reduction, esterification and long chain polymerization. It is also used as a monomer in the preparation of polyglycolic acid and other biocompatible copolymers. Glycolic acid is often included into emulsion polymers, solvents and additives for ink and paint in order to improve flow properties and impart gloss.Other uses :in the textile industry as a dyeing and tanning agent, in food processing as a flavoring agent and as a preservative.
3. Production
Glycolic acid (CAS NO.79-14-1) is prepared by the reaction of chloroacetic acid with sodium hydroxide followed by re-acidification as follows:
ClCH2CO2H + NaOH → HOCH2CO2H + NaCl
In this way, a few million kilograms are produced annually. Other methods, include hydrogenation of oxalic acid and the hydrolysis of the cyanohydrin derived from formaldehyde.but not apparently in use. Glycolic acid can be isolated from natural sources, such as sugar beets, sugarcane, canteloupe, pineapple, and unripe grapes.
Glycolic acid,we can also using an enzymatic biochemical process,compared to traditional chemical synthesis,it produces fewer impurities , requires less energy in production and produces less co-product.
4. Safety
Glycolic acid is a strong irritant depending on the pH levels. Like ethylene glycol, it is metabolized to oxalic acid, which could make it dangerous if ingested.
Moderately toxic by ingestion. A severe eye irritant. A skin and mucous membrane irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes: C
Risk Statements: 34-22
R34: Causes burns.
R22: Harmful if swallowed.
Safety Statements: 26-36/37/39-45-23
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S23: Do not breathe vapour.
RIDADR: UN 3265 8/PG 3
WGK Germany: 1
RTECS: MC5250000
HazardClass: 8
PackingGroup: II
没有评论:
发表评论